Circadian rhythms regulate sleep-wake cycles, metabolism, immune function, and reproduction in mammals. These processes are coordinated by the circadian clock, a biochemical oscillator that integrates physiological input signals with distinct oscillatory phases to regulate rhythms in organismal physiology, behavior, and metabolism. Accumulating evidence indicates that aging in mammals is intricately linked with alterations in circadian rhythms. However, whether and how the circadian machinery directly regulates stem cell aging, especially in primates, remains poorly understood.
Recently, researchers from the Institute of Zoology, Chinese Academy of Sciences, and Sun Yat-sen University have collaborated to uncover a novel role of BMAL1 in regulating primate stem cell senescence by repressing the“jumping gene” LINE1. This study entitled “BMAL1 moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates” was published online in Nucleic Acids Research on March 14, 2022.
BMAL1, a transcription factor that initiates transcriptional-translational feedback loops that drive the oscillation of circadian genes, is an indispensable component of the molecular circadian. So far, the scientific link between BMAL1 and aging is unclear. In this study, researchers found that nuclear BMAL1 was reduced in senescent mesenchymal progenitor cells (MPCs) from both humans and monkeys, indicating that nuclear BMAL1 might have a conserved function in aging regulation in primates. To study the role of BMAL1 in regulating cellular homeostasis, researchers generated BMAL1-deficient human MPCs by CRISPR/Cas9 gene editing, and found that BMAL1 depletion led to accelerated cellular senescence. Interestingly, the function of BMAL1 in counteracting cellular senescence is independent of its C-terminal transactivation domain and thus uncoupled from its circadian activity. The mechanistic investigation demonstrated that BMAL1 interacted with proteins associated with heterochromatin and nuclear lamina, such as KAP1 and Lamin B1, to stabilize heterochromatin and repress LINE1. BMAL1 deficiency led to detachment of genomic lamina-associated domains (LADs) from the nuclear periphery, decreased H3K9me3 occupancy, and increased chromatin accessibility in LADs.
Further, BMAL1 occupies the LINE1 repetitive elements, and its deficiency causes LINE1 de-repression, which in turn activates cGAS-STING proinflammatory pathways. Importantly, blocking LINE1 activity can alleviate BMAL1 deficiency-induced cellular aging, suggesting that LINE1 repression is likely the mechanism through which BMAL1 regulates senescence. Finally, BMAL1 occupancy at LINE1 was reduced during senescence, and targeted BMAL1 deletion in a monkey model also led to LINE1 activation and tissue aging in vivo, indicating a conserved role of BMAL1 in moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates.
For the first time, this study identifies a novel function of BMAL1 independent of its transcriptional activity through which it regulates tissue and stem cell senescence. This research expands our understanding of the biological functions of core circadian clock proteins, sheds new light on an inextricable connection between circadian rhythm and aging regulation, and may provide a novel target for the treatment of aging-associated degenerative disorders in the future.
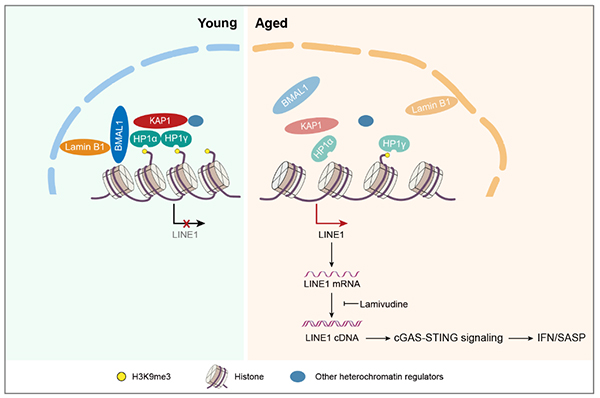
Figure: Dampened BMAL1 occupancy on LINE1 elements leads to its derepression and thus activates cellular senescence through cGAS-STING pathway.
链接:https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkac146/6548307?login=true
( Contact : Guang-Hui Liu , ghliu@ioz.ac.cn )

