Circadian clock is an evolutionarily conserved biological timing system that stabilizes tissue and cellular homeostasis by synchronizing mammalian physiology and behaviors with the external light-dark cycle. Accumulating evidence indicates an inextricable link between the circadian clock and the aging process. Adult stem cell exhaustion or senescence is one of the aging hallmarks and a crucial driving force for human degenerative disorders such as aging-related articular degeneration. However, the role of core circadian clock components in contributing to human stem cell aging has not been reported.
Recently, researchers from the Institute of Zoology and Beijing Institute of Genomics of the Chinese Academy of Sciences have collaborated to uncover a novel role of CLOCK in rejuvenating stem cell senescence and facilitating cartilage regeneration. This study entitled “Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration” was published online in Cell Research on July 31th, 2020.
CLOCK is a component of the core molecular circadian clock, which plays important roles in maintaining circadian rhythm at the molecular/tissue/organismal levels. However, the role of CLOCK in regulating human aging remains largely unknown. In this study, researchers generated CLOCK-deficient human mesenchymal stem cells (hMSCs) by CRISPR/Cas9-mediated gene editing and directed differentiation and found that CLOCK deficiency accelerated hMSC senescence. Mechanically studies further revealed that CLOCK formed a complex with nuclear lamina proteins lamin B1, LBR, and Emerin as well as a heterochromatin-associated protein KAP1 to stabilize heterochromatin and inhibit excessive transcription of repetitive elements, thus contributing to genomic stability and stem cell rejuvenation independent of its transcriptional activation activity. Accordingly, re-introduction of wildtype CLOCK or its transcriptional inactivation mutants effectively delayed the senescence phenotypes of hMSCs. Furthermore, CLOCK was downregulated in replicative-senescent, physiological-senescent and premature aging human mesemchymal stem cells whereas the overexpression of CLOCK effectively reversed the senescence phenotypes of these hMSCs. More importantly, overexpression of CLOCK mitigated the degeneration of articular cartilage, reduced the proportion of senescent cells, increased the level of H3K9me3, suppressed immune response and stimulated cartilage regeneration, thereby alleviating age-related joint degeneration and improving athletic ability in aged mice.
For the first time, this study reveals a novel role of CLOCK in regulating human stem cell aging via heterochromatin stabilization and emphasizes the therapeutic potentials in treating aging-associated degenerative disorders.
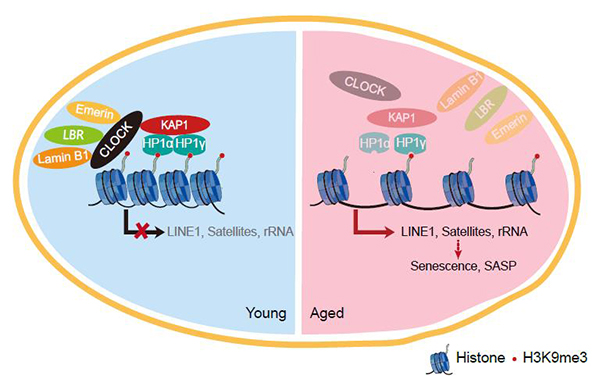
A constructive model describing the role of CLOCK in stabilizing heterochromatin during aging.
(Contact: Guang-Hui Liu, ghliu@ioz.ac.cn)

