| By using genetics, embryo culture and ES cells as the model systems, our researches focus on molecular and cellular basis of early embryonic development and gametogenesis. Research directions: 1)Cell lineage specification of early embryonic development: Early embryonic cells are totipotent, progressively differentiate into pluripotent, and then commit to three germ layer cells. Recently, we have established an efficient in vitro culture (IVC) system to support mammalian blastocysts grow beyond gastrulation. We have also derived a mammalian formative pluripotent stem cells (fPSCs), which is poised for gastrulation. By using these systems, we are investigating genetic and epigenetic controls of early cell lineage commitment during embryonic development. 2) Maternal control of vertebrate early embryogenesis: Prior zygotic genome activation, early embryogenesis is entirely controlled by maternal effect genes. Recently, we have identified oocyte-embryo specific expressed SCMC that is essential for mammalian early embryogenesis. Currently we focus on the molecular mechanisms of subcortical maternal complex (SCMC). In addition, we investigate maternal DNA damage repair and maternal RNA degradation during vertebrate maternal-to-zygotic transition. 3) Molecular mechanism of mammalian gametogenesis: We found some alternative pre-mRNA splicing factors specifically expresses and play important role in mouse gametogenesis. Currently, we investigate how these factors regulate mouse gametogenesis by using mouse genetic and RNA-sequence approaches. 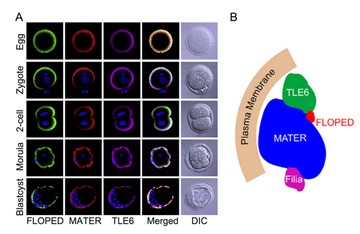
Localization (A) and a model (B) of the SCMC (Li et al., Dev. Cell, 2008).
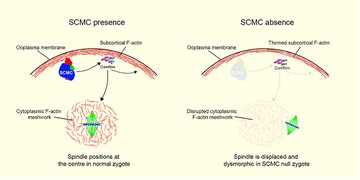
The SCMC regulates the symmetric division through F-actin in mouse zygote (Yu et al., Nat. Commun., 2014).
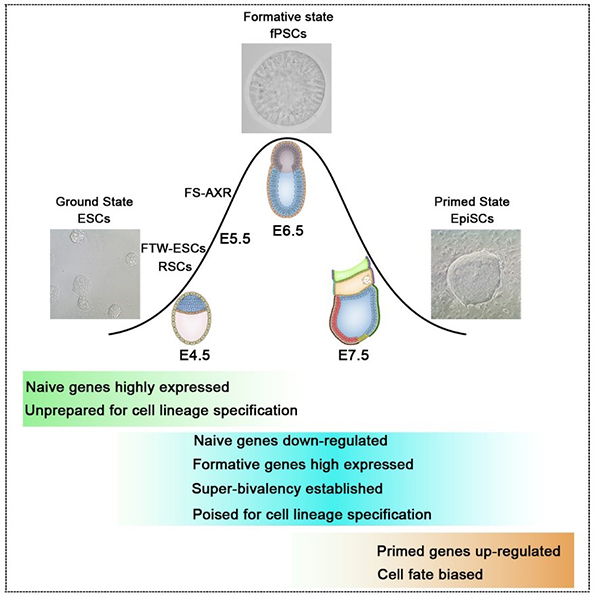
Formative pluripotent stem cells (fPSCs), (Wang et al., Cell Res, 2021).
| Plain English:
We are interesting in cell lineage specification of early embryonic development and also interesting in the genes specifically expressed in germ cell that play important roles during early embryonic development and gametogenesis. Our searches will contribute to the clinic application of stem cells, human reproductive disorders and contraception. Selected publications: - Chi Q, Ou G, Qin D, Han Z, Li J, Xiao Q, Gao Z, Xu C, Qi Q, Liu Q, Liu S, Li J, Guo L, Lu Y, Chen J, Wang X, Shi H, Li L*, Deng D*. Structural basis of the subcortical maternal complex and its implications in reproductive disorders. Nature Structural & Molecular Biology. 2024 Jan;31(1):115-124. doi: 10.1038/s41594-023-01153-x. Epub 2024 Jan 4
- Nie X, Xu Q, Xu C, Chen F, Wang Q, Qin D, Wang R, Gao Z, Lu X, Yang X, Wu Y, Gu C, Xie W*, Li L*. Maternal TDP-43 interacts with RNA Pol II and regulates zygotic genome activation. Nature Communications. 2023 Jul 17 (14: 4275). https://doi.org/10.1038/s41467-023-39924-1.
- Quan Y, Wang M, Xu C, Wang X, Qin D, Lin Y, Lu X, Lu F*, Li L*. Cnot8 eliminates naïve regulation networks and is essential for naïve-to-formative pluripotency transition. Nucleic Acids Research. 2022 May 6;50(8):4414-4435. doi: 10.1093/nar/gkac236.
- Xiong Z, Xu K, Lin Z, Kong F, Wang Q, Quan Y, Sha Q, Li F, Zou Z, Liu L, Ji S, Chen Y, Zhang H, Fang J, Yu G, Liu B, Wang L, Wang H, Deng H, Yang X, Fan H, Li L*, Xie W*. Ultrasensitive Ribo-seq reveals translational landscapes during oocyte-to-embryo transition and early development. Nature Cell Biology. 2022. doi:10.1038/s41556-022-00928-6.
- Wang X, Xiang Y, Yu Y, Wang R, Zhang Y, Xu Q, Sun H, Zhao Z, Jiang X, Wang X, Lu X, Qin D, Quan Y, Zhang J, Shyh-Chang N, Wang H, Jing N, Xie W*, Li L*. Formative pluripotent stem cells show features of epiblast cells poised for gastrulation. Cell Research. 2021 May; 31(5): 526-541. doi.org/10.1038/s 41422-021-00477-x.
- Ma H, Zhai J, Wan H, Jiang X, Wang X, Wang L, Xiang Y, He X, Zhao Z, Zhao B, Zheng P*, Li L*, Wang H*. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. 2019 Oct 31. pii: eaax7890. doi: 10.1126/science.aax7890.
- Qin D, Gao Z, Xiao Y, Zhang X, Ma H, Yu X, Nie X, Fan N, Wang X, OuYang Y, Sun Q, Yi Z, Li L*. The subcortical maternal complex protein Nlrp4f is involved in cytoplasmic lattice formation and organelle distribution. Development. 2019 Oct 18; 146(20). pii: dev183616. doi: 10.1242/dev.183616.
- Lu X, Gao Z, Qin D, Li L*. A maternal functional module in the mammalian oocyte-to-embryo transition. Trends in Molecular Medicine. 2017 Nov; 23 (11) 1014–1023.
- Liu W, Wang F, Xu Q, Shi J, Zhang X, Lu X, Gao Z, Ma H, Zhao Z, Duan E, Gao F, Gao S*, Yi Z*, Li L*. BCAS2 is involved in alternative mRNA splicing in spermatogonia and the transition to meiosis. Nature Communications. 2017 Jan 27; 8:14182.
- Xu Q, Wang F, Xiang Y, Zhang X, Zhao Z, Gao Z, Liu W, Lu X, Liu Y, Yu X, Wang H, Huang J, Yi Z, Gao S*, Li L*. Maternal BCAS2 protects genomic integrity in mouse early embryonic development. Development. 2015 Nov 15; 142 (22):3943-53.
- Yu X, Yi Z, Gao Z, Qin D, Zhai Y, Chen X, Ou-Yang Y, Wang Z, Zheng P, Zhu M, Wang H, Sun QY, Dean J*, Li L*. The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics. Nature Communications. 2014 Sep 11; 5:4887.
- Li L*, Baibakov B and Dean J. A subcortical maternal complex essential for pre-implantation mouse embryogenesis. Developmental Cell. 2008 Sep; 15(3): 416-25.
|

