| Traumatic brain injury (TBI) is the leading cause of death and disability worldwide, especially in children and young adults. TBI patients are at increased risk of developing a subsequent neurodegenerative or mental disorder or both. Unfortunately, there are no effective medications currently available for prevention and treatment of TBI. Our research focuses on understanding the pathological mechanisms of neurodegeneration induced by TBI, and developing early diagnostic and treatment approaches for cognitive problem and mental illness in brain trauma. Over the past few years, we have constructed a database of gene expression profiles of neural stem cells and microglia under different time windows of brain trauma, and identified dozens of genes that might be closely related to neurogenesis, cell survival, synaptic transmission, synaptic plasticity, neuroinflammation, or phenotypic transformation of microglia. We also have developed several techniques to generate microglia, neural progenitor cells, excitatory neurons, inhibitory neurons and brain organoids from human pluripotent stem cells. We have been trying to comprehensively use multidisciplinary methods such as genetics, molecular biology, biochemistry, cell biology, bioinformatics, behavioral science, electrophysiology, and nanomaterial, taking into account both animal models and humanized neural models, to discover novel therapeutic targets for neurodegenerative and mental disorders, and to develop approaches to promote neural regeneration. 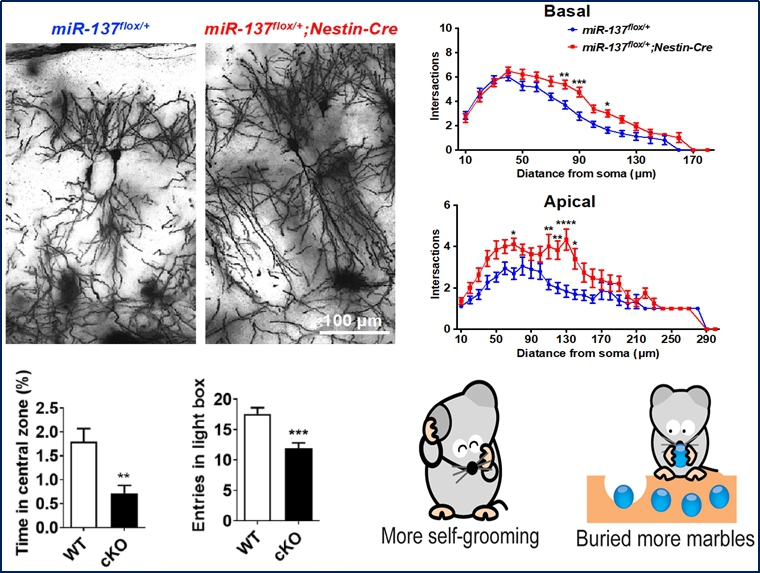 We found that loss of MIR137, a mental illness risk gene, has typical anxiety, cognitive and social disabilities in mice. Pde10a and Ezh2 were identified as miR-137’s downstream targets that regulate cognitive and mood-related behaviors (Nature Neurosci. 2018; Front Mol Neurosci. 2019; Exp Neurobiol. 2020). We found that loss of MIR137, a mental illness risk gene, has typical anxiety, cognitive and social disabilities in mice. Pde10a and Ezh2 were identified as miR-137’s downstream targets that regulate cognitive and mood-related behaviors (Nature Neurosci. 2018; Front Mol Neurosci. 2019; Exp Neurobiol. 2020).
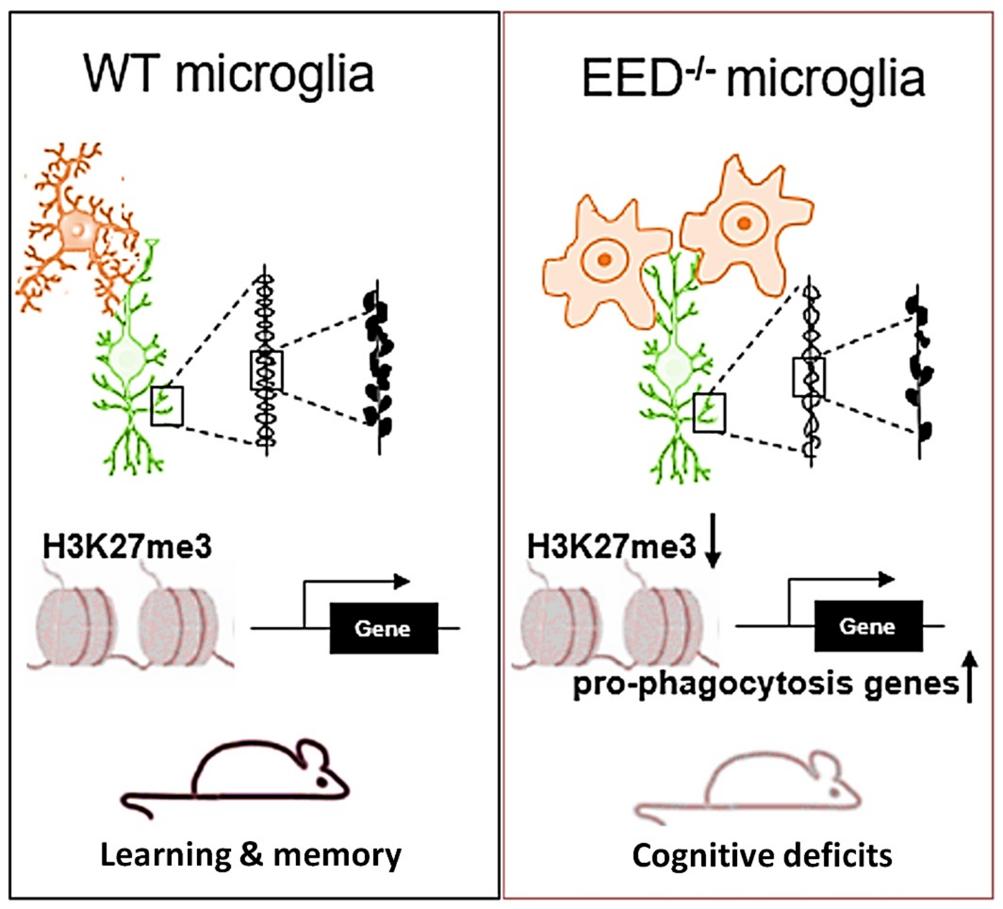 We reported for the first time that microglial EED is important for having normal synapse pruning and cognitive functions in the mouse brain, which implicates EED mutations in the pathologies of cognitive disorders (Mol Psychiatry. 2022). We reported for the first time that microglial EED is important for having normal synapse pruning and cognitive functions in the mouse brain, which implicates EED mutations in the pathologies of cognitive disorders (Mol Psychiatry. 2022).
| Plain english:
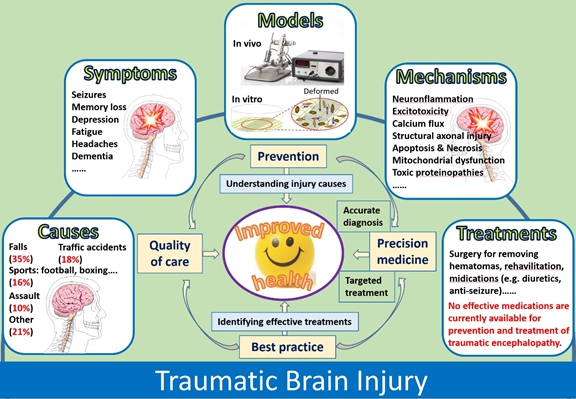
Traumatic brain injury (TBI) is the damage to the brain due to sudden trauma, either by penetrating or, more commonly, by closed head injury. TBI is a major public health and socioeconomic problem around the world. TBI survivors usually develop many troubling symptoms, such as seizures, pain, body motor dysfunction, memory loss, and psychological and social cognitive defects. Recently, some evidences indicate that TBI may interact with other factors to trigger neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease.
Our research interests include, but are not limited to: 1) characterizing the effects of TBI on endogenous neurogenesis and gliogenesis; 2) identifying both genetic and epigenetic factors that might be associated with a complex cascade of molecular, cellular and immune responses, resulting in abnormal neurogenesis, neuroinflammation, neuronal injury, and cell death; 3) elucidating the mechanism of microglial transformation; and 4) developing approaches to promote neural regeneration. Selected publications: - Wang YY, Deng YS, Dai SK, Mi TW, Li RY, Liu PP, Liu C, He BD, He XC, Du HZ, Yang HC, Tang Y, Liu CM*, Teng ZQ*. Loss of microglial EED impairs synapse density, learning, and memory. Molecular Psychiatry 2022, doi: 10.1038/s41380-022-01576-w.
- He XC, Wang J, Du HZ, Liu CM*, Teng ZQ*. Intranasal Administration of Agomir-let-7i Improves Cognitive Function in Mice with Traumatic Brain Injury. Cells . 2022, 11(8): 1348.
- Liu XT, Liu CM*, Teng ZQ*. Mouse model of voluntary movement deficits induced by needlestick injuries to the primary motor cortex. Journal of Neuroscience Methods 2022, 365: 109380.
- Dai SK, Liu PP, Du HZ, Liu X, Xu YJ, Liu C, Wang YY, Teng ZQ*, Liu CM*. Histone crotonylation regulates neural stem cell fate decisions by activating bivalent promoters. EMBO Reports 2021, 22 (10): e52023.
- Liu C#, Dai SK#, Shi RX, He XC, Wang YY, He BD, Sun XW, Du HZ, Liu CM*, Teng ZQ*. Transcriptional profiling of microglia in the injured brain reveals distinct molecular features underlying neurodegeneration. Glia 2021, 69 (5): 1292-1306.
- Wang ZM, Liu C, Wang YY, Deng YS, He XC, Du HZ, Liu CM*, Teng ZQ*. SerpinA3N deficiency deteriorates impairments of learning and memory in mice following hippocampal stab injury. Cell Death Discovery 2020, 6 (1): 1-11.
- Tang QY, Zhang SF, Dai SK, Liu C, Wang YY, Du HZ, Teng ZQ*, Liu CM*. UTX regulates human neural differentiation and dendritic morphology by resolving bivalent promoters. Stem Cell Reports 2020, 15 (2): 439-453.
- Mi TW, Sun XW, Wang ZM, Wang YY, He XC, Liu C, Zhang SF, Du HZ, Liu CM*, Teng ZQ*. Loss of MicroRNA-137 Impairs the Homeostasis of Potassium in Neurons via KCC2. Experimental Neurobiology 2020, 29(2): 138-149.
- Liu C#, Dai SK#, Sun Z#, Wang Z, Liu PP, Du HZ, Yu S*, Liu CM*, Teng ZQ*. GA-binding protein GABPβ1 is required for the proliferation of neural stem/progenitor cells. Stem Cell Res earch 2019, 39: 101501.
- Yan HL#, Sun XW#, Wang ZM#, Liu PP#, Mi TW, Liu C, Wang YY, He XC, Du HZ, Liu CM*, Teng ZQ*. MiR-137 Deficiency Causes Anxiety-Like Behaviors in Mice. Frontiers in Molecular Neuroscience 2019, 12: 260.
- Cheng Y#, Wang ZM#, Tan W#, Wang X#, Li Y, Bai B, Li Y, Zhang SF, Yan HL, Chen ZL, Liu CM, Mi TW, Xia S, Zhou Z, Liu A, Tang GB, Liu C, Dai ZJ, Wang YY, Wang H, Wang X, Kang Y, Lin L, Chen Z, Xie N, Sun Q, Xie W, Peng J, Chen D*, Teng ZQ*, Jin P*. Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat ure Neuroscience 2018, 21(12): 1689-1703.
- Duan RS#, Tang GB#, Du HZ#, Hu YW, Liu PP, Xu YJ, Zeng YQ, Zhang SF, Wang RY, Teng ZQ, Liu CM*. Polycomb protein family member CBX7 regulates intrinsic axon growth and regeneration. Cell Death and Differentiation 2018, 25(9): 1598-1611.
- Liu PP, Xu YJ, Teng ZQ, Liu CM*. Polycomb Repressive Complex 2: Emerging Roles in the Central Nervous System. Neuroscientist 2018, 24(3): 208-220.
- Liu PP, Tang GB, Xu YJ, Zeng YQ, Zhang SF, Du HZ, Teng ZQ*, Liu CM*. MiR-203 Interplays with Polycomb Repressive Complexes to Regulate the Proliferation of Neural Stem/Progenitor Cells. Stem Cell Reports 2017, 9(1): 190-202.
- Chen R#, Zhang J#, Fan N#, Teng ZQ#, Wu Y#, Yang H, Tang YP, Sun H, Song Y, Chen C*. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 2013, 155(5): 1154-1165.
- Liu C#, Teng ZQ#, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X*. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell 2010, 6(5): 433-44.
|

