| We aimed to study the physiological mechanisms underlying placental trophoblast differentiation and the correlation with pregnancy complications and diseases, with a focus on the key events including trophoblast primary syncytialization (the first cell-cell fusion event after fertilization), secondary syncytialization (a continuous fusion event creating the largest syncytia across species) and invasion/migration by using placenta-specific knockout mice, live-cell imaging, high-throughput proteomics, single cell RNA sequencing and various cell or tissue models. we focus on investigating the pathological dysfunction and physiological aging of the ovary. The pathogenesis and treatment of ovarian dysfunction, especially premature ovarian insufficiency, are of interest to us. We are also studying the mechanisms that regulate normal ovarian physiological aging to find the secret of senility. In addition, building a complete data model of follicle number with age is an important challenge in understanding ovarian reproductive aging. The aims of these works are to understand the ovarian physiology and to promote the theoretical development and clinical transformation of the treatment of ovarian diseases and fertility recovery in women. Primate’s early embryogenesis is another subject we focused on, especially at the post-implantation stage. Due to the inaccessibility of post-implantation embryos, the limitation of detection techniques and research ethic, developmental events of primate embryos still maintains poorly understand. Here, we established an in vitro culture (IVC) system that supports the continuous development of cynomolgus monkey blastocysts beyond early gastrulation and to 20 days post fertilization. The IVC embryos highly recapitulated the key events of in vivo early post-implantation development without the maternal supporting This study provided important theoretical basis for human embryos gastrulation and related diseases. Based on these research background, we will further optimize the long-term IVC system, and promote primate (non-human) embryos to more advanced developmental stage to discuss more possibilities of life. 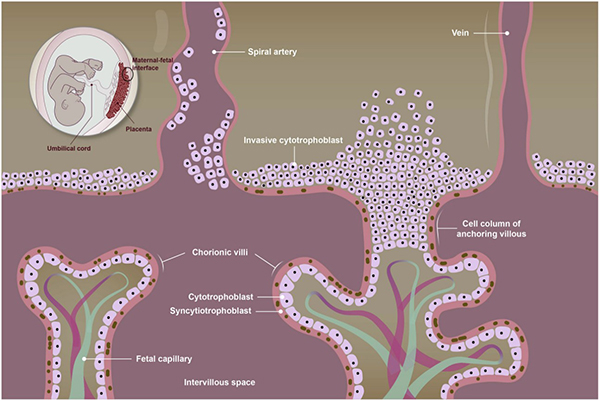
Xiao Z, et al. Progress in deciphering trophoblast cell differentiation during human placentation. Current Opinion in Cell Biology. 2020
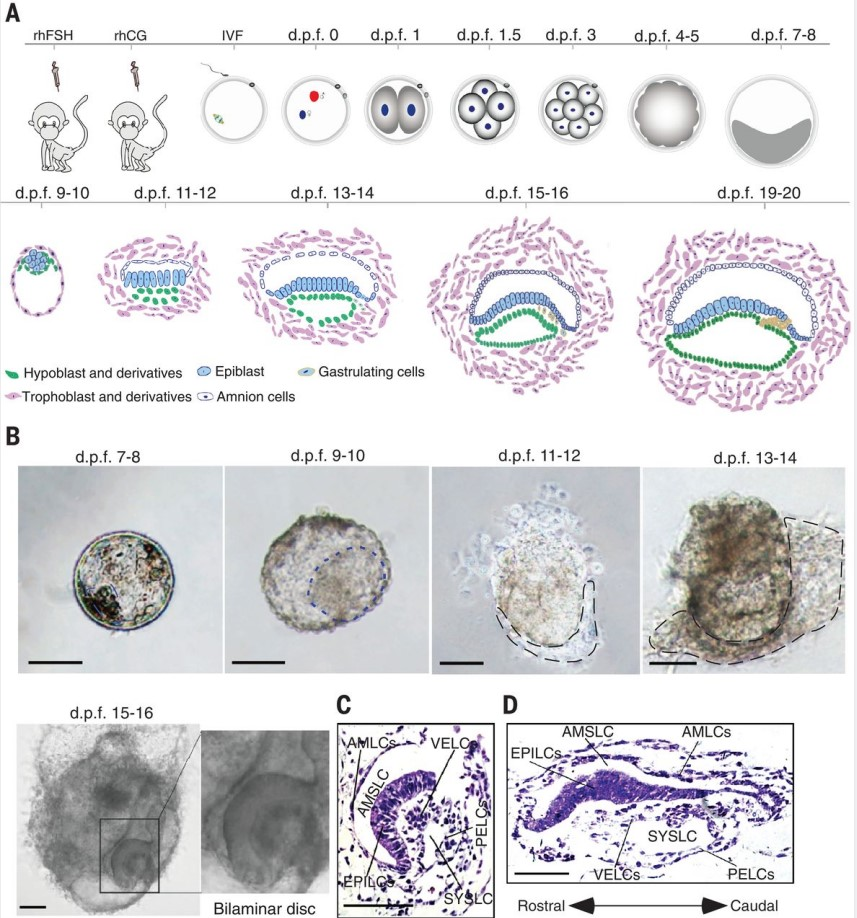
Ma H, et al. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. 2019
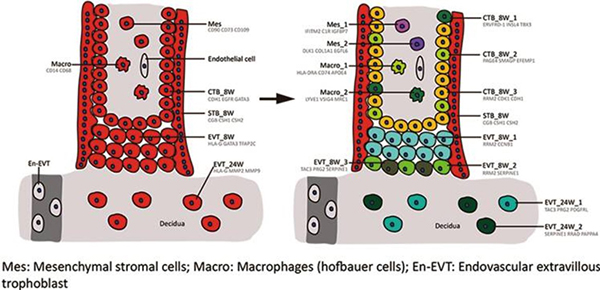
Liu Y, et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Research, 2018
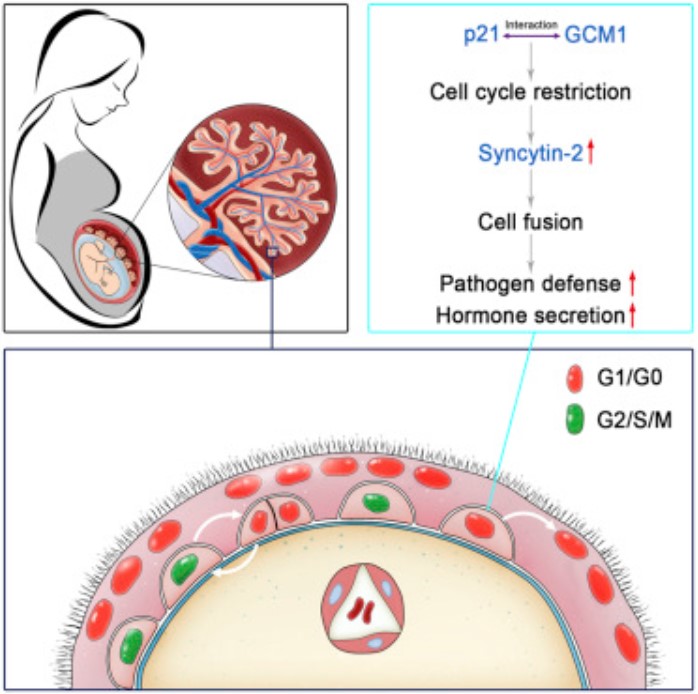
Lu X, et al. Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Reports. 2017
| Plain english:
Our group is committed to the study of female reproductive health. We focused on the molecular mechanism of early embryonic development, placental development and pregnancy maintenance, and the relationship between ovarian function and pregnancy outcome: (1) early embryonic development is closely related to successful pregnancy. We used human, cynomolgus monkey and mouse models to study the pathogenesis of early embryonic development, placental development and related diseases (2) Normal mammalian folliculogenesis is a reliable guarantee for the periodic discharge of healthy and fertile eggs. Abnormal follicular activation can lead to reproductive diseases such as premature ovarian failure (POF) and polycystic ovary syndrome (PCOS), which seriously threaten women's health. We used mouse and primate models as well as clinical human disease resources to reveal the molecular mechanisms of follicular development and fertility maintenance. Selected publications: - Yu D#, Wan H#, Tong C#, Guang L#, Chen G#, Su J#, Zhang L, Wang Y, Xiao Z, Zhai J, Ma W, Liang K, Liu T, Wang Y, Peng Z, Luo L, Yu R, Li W*, Qi H*, Wang H*, Shyh-Chang N*. A multi-tissue metabolome atlas reveals core signatures and critical nodes of metabolic reprogramming in female primates during pregnancy. Cell. 2024, 187, 764-781 e714.1.
- Wang M#, Liu Y#, Sun R#, Liu F#, Li J, Yan L, Zhang J, Xie X, Li D, Wang Y, Li S, Zhu X, Li R*, Lu F*, Xiao Z*, Wang H*. Single-nucleus multi-omic profiling of human placental syncytiotrophoblasts identifies cellular trajectories during pregnancy. Nat Genet. 2024, 56, 294-305.
- Wang Y#, Deng W#, Lee D#, Yan L#, Lu Y, Dong S, Huntoon K, Antony A, Li X, Ye R, Zhao Y, Zhao F, Schrank BR, Ha J, Kang M, Yang M, Gong P, Lorenzi PL, Tan L, Gallup TD, Tang SK, Yang Z, Li J, Sanford NN, Wang H*, Kim BYS*, Jiang W*. Age-associated disparity in phagocytic clearance affects the efficacy of cancer nanotherapeutics. Nat Nanotechnol. 2024,19, 255-263.
- Wu H#, Zhai J#, Wang H*. Unraveling the function of FGF signaling in human hypoblast specialization. Cell Stem Cell. 2024, 31(7):945-946. (comments)
- Yu X#, Wu H#, Su J#, Liu X, Liang K, Li Q, Yu R, Shao X, Wang H*, Wang YL*, Shyh-Chang N*. Acetyl-CoA metabolism maintains histone acetylation for syncytialization of human placental trophoblast stem cells. Cell Stem Cell. 2024, 31(9):1280-1297.e7.
- Wu X#, Zhai J#, Li Q#, Wang H*. The in vitro culture of mammalian embryos. Nat Method.2023, 20, 1855-1858. (comments)
- Wu H#, Huang XY#, Sun MX#, Wang Y#, Zhou HY#, Tian Y#, He BJ, Wu AP, Wang H*, Qin CF*. Zika virus targets human trophoblast stem cells and prevents syncytialization in placental trophoblast organoids. Nat Commun. 2023, 14, 5541.
- Zhai J#, Xu Y#, Wan H#, Yan R#, Guo J#, Skory R#, Yan L, Wu X, Sun F, Chen G, Zhao W, Yu K, Li W*, Guo F*, Plachta N*, Wang H*. Neurulation of the cynomolgus monkey embryo achieved from 3D blastocyst culture. Cell. 2023, 186(10). cover story.
- Jiang X #, Zhai J #, Xiao Z#, Wu X#, Zhang D#, Wan H, Xu Y, Qi L, Wang M, Yu D, Liu Y, Wu H, Sun R, Xia S, Yu K, Guo J, Wang H*. Identifying a dynamic transcriptomic landscape of the cynomolgus macaque placenta spanning during pregnancy at single-cell resolution. Dev Cell. 2023 Apr; 58:806-821.
- Jiang X#*, Wang Y# , Xiao Z, Yan L, Guo S, Wang Y, Wu H, Zhao X, Lu X*, Wang H*. A differentiation roadmap of murine placentation at single-cell resolution. Cell Discov. 2023 Mar;9(1):30.
- Zhai J#, Guo J#, Wan H#, Qi L#, Liu L#, Xiao Z, Yan L, Schmitz DA, Xu Y, Yu D, Wu X, Zhao W, Yu K, Jiang X*, Guo F*, Wu J*, Wang H*. Primate gastrulation and early organogenesis at single-cell resolution. Nature. 2022 Dec;612(7941):732-738.
- Zhou JJ#, Jiang XX#, Wu HW#, Zhang LJ, Chen M, Chen M, Shen ZM, Guo XD*, Wang H*, Gao F*, Dissecting the fate of 1 Foxl2-expressing cells in fetal ovary using lineage tracing and Single-Cell Transcriptomics. Cell Discov. 2022 Dec;8(1):139.
- Yan R#, Cheng X#, Gu C#, Xu YH#, Long X#, Zhai JL, Sun FY, Qian JJ, Du YR, Wang H*, Guo F*, Dynamics of DNA hydroxymethylation and methylation during mouse embryonic and germline development. Nat Genet. 2023 Jan, 55(1):130-143.
- Li Q#, Wu H#, Wang Y, # Wang H*, Current understanding in deciphering trophoblast cell differentiation during human placentation. Biol Reprod. 2022, 107(1):317-326. Review
- Gu Z#, Guo J#, Zhai J#, Feng G, Wang X, Gao Z, Li K, Ji S, Wang L, Xu Y, Chen X, Wang Y, Guo S, Yang M, Li L,Hua H, Jiang L, Wen Y, Wang L, Hao J, Li W, Wang S,* Wang H*, Gu Q*. A Uterus-Inspired Niche Drives Blastocyst Development to the Early Organogenesis. Adv Sci. 2022,10.1002.
- Tu W#, Ni D#, Yang H#, Zhao F#, Yang C#, Zhao X#, Guo Z#, Yu K, Wang J, Hu Z, Chen Z, Zhao Y, Wang Z, Gao F, Yan L*, Yang X*, Zhu L*, Wang H*. Deciphering the dynamics of the ovarian reserve in cynomolgus monkey through a quantitative morphometric study. Sci Bull. 2022, 67(18): 1854-1859.
- Wang Y#, Jiang X#, Jia L#, Wu X#, Wu H#, Wang Y, Li Q, Yu R, Wang H*, Xiao Z*, Liang X*. A Single-Cell Characterization of Human Post-implantation Embryos Cultured In Vitro Delineates Morphogenesis in Primary Syncytialization. Front Cell Dev Biol. 2022 Jun 15;10:835445.
- Wang Y#, Wu H#, Jiang X#, Jia L#, Wang M, Rong Y, Chen S, Wang Y, Xiao Z*, Liang X*, Wang H*. LMNA Determines Nuclear Morphology During Syncytialization of Human Trophoblast Stem Cells. Front Cell Dev Biol. 2022 Apr 11;10:836390.
- Zhai J#, Xiao Z#, Wang Y#, Wang H*. Human embryonic development: from peri-implantation to gastrulation. Trends Cell Biol. 2022 Jan;32(1):18-29. Review
- Liang G#, Zhou C#, Jiang X#, Zhang Y, Huang B, Gao S, Kang Z, Ma D, Wang F, Gottgens B*, Wang H*, Han JJ*, Liu F*. De novo generation of macrophage from placenta-derived hemogenic endothelium. Dev Cell. 2021 Jul 26;56(14):2121-2133.
- Wu H#, Liao S#*, Wang Y#, Guo M#, Lin X, Wu J, Wang R, Lv D, Wu D, He M, Hu B, Long R, Peng J, Yang H, Yin H, Wang X, Huang Z, Lan K, Zhou Y, Zhang W, Xiao Z, Zhao Y*, Deng D*, Wang H*. Molecular evidence suggesting the persistence of residual SARS-CoV-2 and immune responses in the placentas of pregnant patients recovered from COVID-19. Cell Prolif. 2021 Sep;54(9):e13091.
- Yan R#, Gu C#*, You D, Huang Z, Qian J, Yang Q, Cheng X, Zhang L##, Wang H##, Wang P*, Guo F*. Decoding dynamic epigenetic landscapes in human oocytes using single-cell multi-omics sequencing. Cell Stem Cell. 2021, 28(9):1641-1656.e7.(##Senior author)
- Li X#, Wang Y#, Ma R#, Liu X, Song B, Duan Y, Guo J, Feng G, Cui T, Wang L, Hao J*, Wang H*, Gu Q*. Reconstruction of functional uterine tissues through recellularizing the decellularized rat uterine scaffolds by MSCs in vivo and in vitro. Biomed Mater. 16 (2021) 035023.
- Qu Y#, Chen Q#, Guo S#, Ma C, Lu Y, Shi J, Liu S, Zhou T, Noda T, Qian J, Zhang L, Zhu X, Lei X, Cao Y, Li W, Li W, Plachta N, Matzuk MM, Ikawa M, Duan *E, Zhang Y*, Wang H*. Cooperation-based sperm clusters mediate sperm oviduct entry and fertilization. Protein Cell. 2021 Oct;12(10):810-817.
- Zhao Y#, Ma J#, Yi P#, Wu J#, Zhao F, Tu W, Liu W, Li T, Deng Y, Hao J*, Wang H*, Yan L*. Human umbilical cord mesenchymal stem cells restore the ovarian metabolome and rescue premature ovarian insufficiency in mice. Stem Cell Res Ther. 2020 Nov 4,11(1),466.
- Yan L#, Wu Y#, Li L#, Wu J#, Zhao F, Gao Z, Liu W, Li T, Fan Y*, Hao J*, Liu J*, Wang H*. Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell Prolif. 2020, 53(12), e12938.
- Xiao Z#, Yan L#, Liang X*, Wang H*. Progress in deciphering trophoblast cell differentiation during human placentation. Curr Opin Cell Biol. 2020, 18(67), 86-91. (invited review)
- Lim HYG, Alvarez YD, Gasnier M, Wang Y, Tetlak P, Bissiere S, Wang H, Biro M, Plachta N*. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature. 2020, 585(7825), 404-409.
- Ma H#, Zhai J#, Wan H#, Jiang X, Wang X, Wang L, Xiang Y, He X, Zhao ZA, Zhao B, Zheng P*, Li L*, Wang H*. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science.2019, 366(6467): eaax7890.
- Guo S#, Cui X#, Jiang X#, Duo S, Li S, Gao F*, Wang H*. Tracing the origin of the placental trophoblast cells in mouse embryo development. Biol Reprod. 2020 Mar 13;102(3):598-606.
- Wang R#, Yu R#, Zhu C, Lin HY, Lu X*, Wang H*. Tubulin detyrosination promotes human trophoblast syncytium formation. J Mol Cell Biol. 2019, 11(11), 967-978. (cover story)
- Ma J#, Wu J#, Han L, Jiang X, Yan L, Hao J*, Wang H*. Comparative analysis of mesenchymal stem cells derived from amniotic plate under serum-free condition. Stem Cell Res Ther. 2019, 10(1), 19.
- Liu Y#, Fan X#, Wang R#, Lu X#, Dang YL, Wang H, Lin HY, Zhu C, Ge H, Cross JC*, Wang H* Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 2018, 28(8), 819-832.
- Jiang X, Du MR, Li M, Wang H*. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol Immunol. 2018, 15(12), 1027-1037.
- Chang WL#, Liu YW#, Dang YL#, Jiang XX, Xu H, Huang X, Wang YL, Wang H, Zhu C, Xue LQ, Lin HY, Meng W*, Wang H*. Plac8, a new marker for human interstitial extravillous trophoblast cells, promotes their invasion and migration. Development. 2018, 145(2), dev148932.
- Lu X#, Wang R#, Zhu C, Wang H, Lin HY, Gu Y, Cross JC*, Wang H*. Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Reports. 2017, 21(5), 1150-1159.
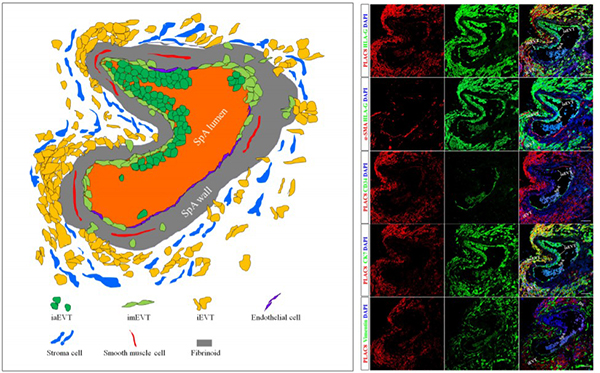
Chang WL, et al. Plac8, a new marker for human interstitial extravillous trophoblast cells, promotes their invasion and migration. Development, 2018
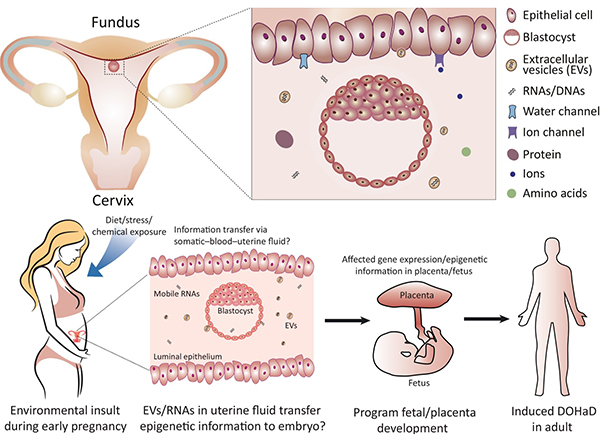
Zhang Y, et al. Uterine Fluid in Pregnancy: A Biological and Clinical Outlook. Trends in Molecular Medicine. 2017
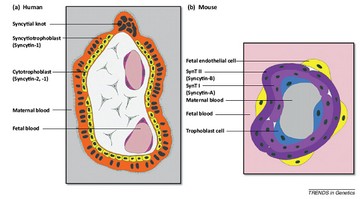
Aguilar PS, et al. Genetic basis of cell–cell fusion mechanisms. Trends Genet, 2013
|

