| The onset of mammalian pregnancy is characterized by the recognition and adhesion of trophoblast cells from the blastocyst to the endometrium, followed by their invasion into the latter. The formation of the placenta is a pivotal event during this process. Properly regulated invasion into the uterine stroma and remodeling of spiral arteries by placental trophoblasts facilitate the successful establishment of utero-placental circulation. Dysfunction in the regulatory network governing trophoblast activities can result in significant clinical complications, including preeclampsia, recurrent miscarriages, and fetal growth restriction. Such disorders impose substantial challenges for both maternal and perinatal healthcare, potentially impacting the short-term and long-term health of affected mothers and offspring. To date, the human placenta remains a largely unexplored "black box", with the regulatory mechanisms underpinning placental development and maternal adaptation to pregnancy remaining largely elusive. There is an urgent need for advancements in the prediction, prevention, and treatment of placenta-associated pregnancy diseases. Guided by pressing scientific inquiries and significant national requirements, Dr. Wang’s Lab is dedicated to exploring the regulatory mechanisms of placental development and pregnancy maintenance. Additionally, it investigates the pathogenesis of major pregnancy-related diseases such as gestational hypertension and recurrent spontaneous abortion. The ultimate goal is to elucidate the intricacies of human pregnancy health, thereby establishing a robust scientific basis for the formulation of predictive and interventional strategies for pregnancy diseases. Dr. Wang's lab has made significant contributions to understanding the metabolic and epigenetic regulatory mechanisms that govern human trophoblast stem cell fate determination and functional characteristics. They identified the role of mTOR-TFEB signaling in mediating trophoblast cell syncytialization, enabling adaptation to environmental nutritional stress via macropinocytosis. Their research elucidated protein glycosylation modification as key factors in trophoblast cell differentiation. They explored the immunoregulatory function of endovascular trophoblast cells and the dedifferentiation process of smooth muscle cells during uterine spiral artery remodeling. Additionally, they provided a comprehensive analysis of the cellular and molecular regulatory networks involved in pregnancy adaptation at the maternal-fetal interface. They developed a humanized mouse model to study dNK cells homing to the uterus, highlighting the crucial role of CD56brightCD39+ dNK cells in maintaining pregnancy. In collaboration with multicenter clinical units, they established prospective resource libraries for various pregnancy diseases, identifying early predictive markers for preeclampsia and other conditions. The study also delved into the molecular pathways of multicellular interactions at the maternal-fetal interface, revealing disruptions in maternal pregnancy adaptation linked to diseases such as preeclampsia, fetal growth restriction, and recurrent miscarriage. Dr’ Wang has published over one hundred peer-reviewed papers in journals including Cell Stem Cell, Natl Sci Rev, PNAS, and others. 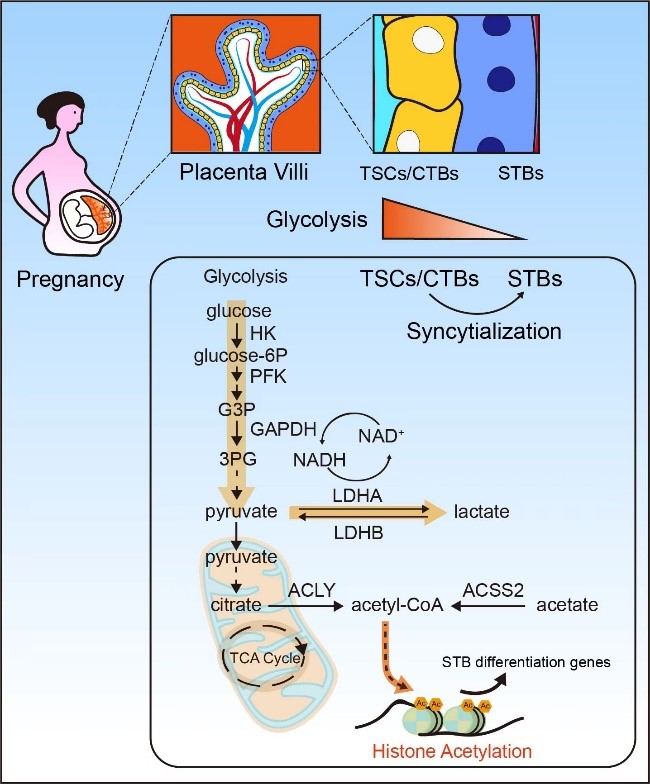
The metabolic and epigenetic regulatory mechanisms underlying the fate determination and functional characteristics of human trophoblast stem cells (Cell Stem Cell 2024)
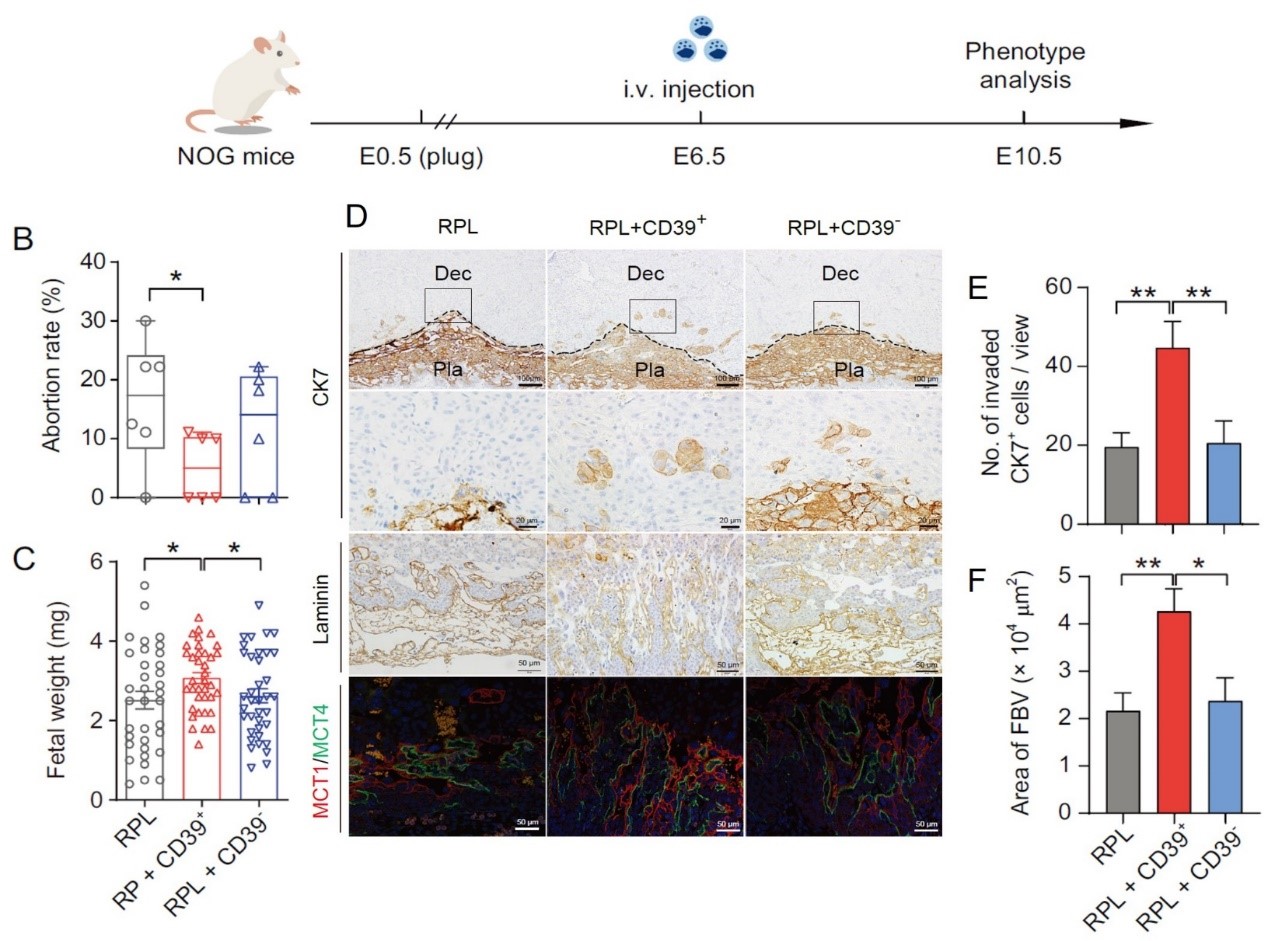
The mechanism of CD56brightCD39+ dNK cells in maintaining pregnancy revealed by a humanized mouse model for dNK cell homing to the uterus (Natl Sci Rev 2024)
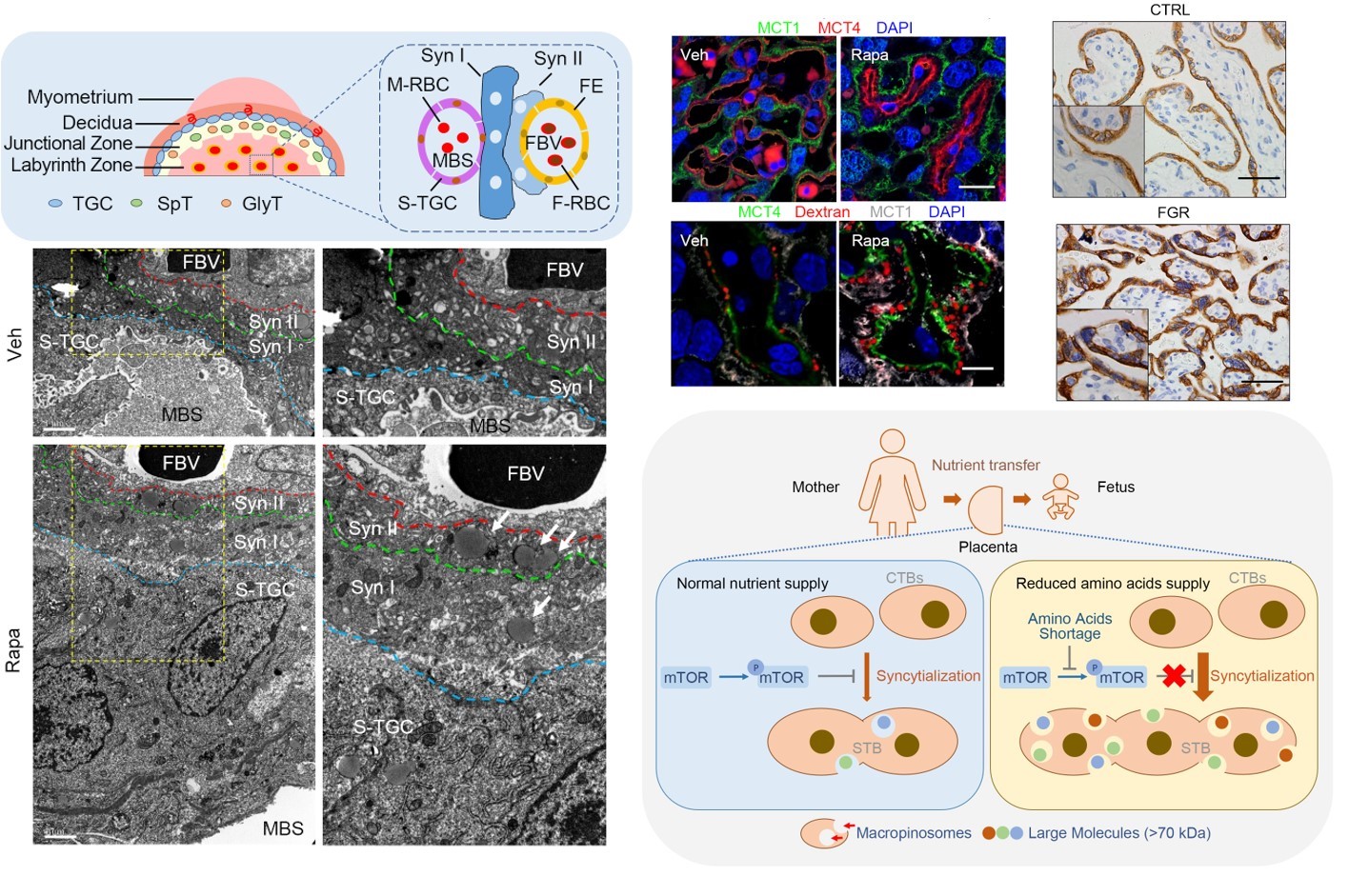
The mTOR-TFEB axis precisely regulates trophoblast syncytialization and promotes macropinocytosis to adapt to amino acid deficiency (PNAS 2021, 2024)
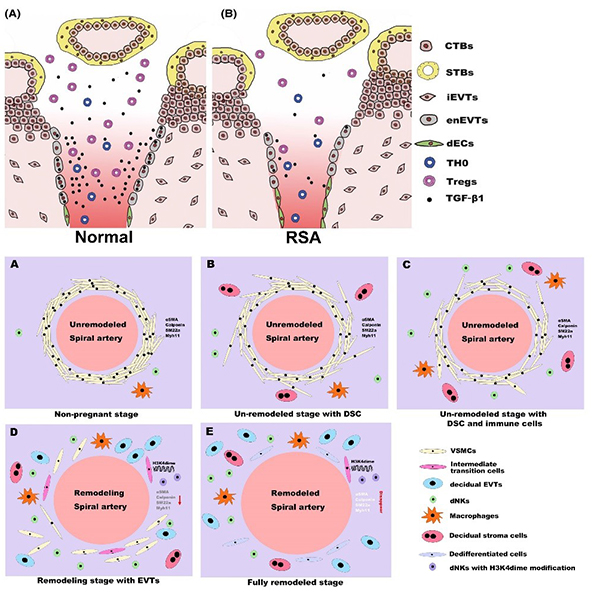
The immunoregulatory role of vascular trophoblast cells and the dedifferentiation pathway of smooth muscle cells during the uterine spiral arteries remodeling (Biol Reprod 2021, Cell Prolif 2020)
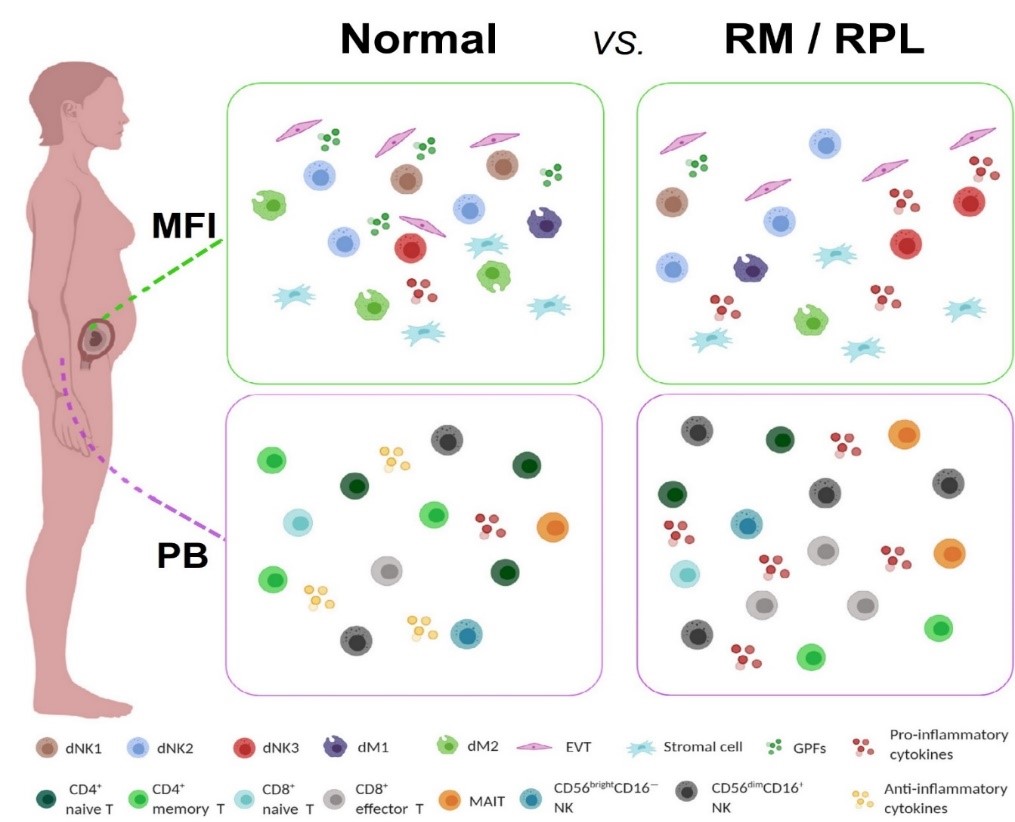
Single-Cell Immune Landscape of Human Recurrent Miscarriage (Genomics Proteomics Bioinformatics 2021)
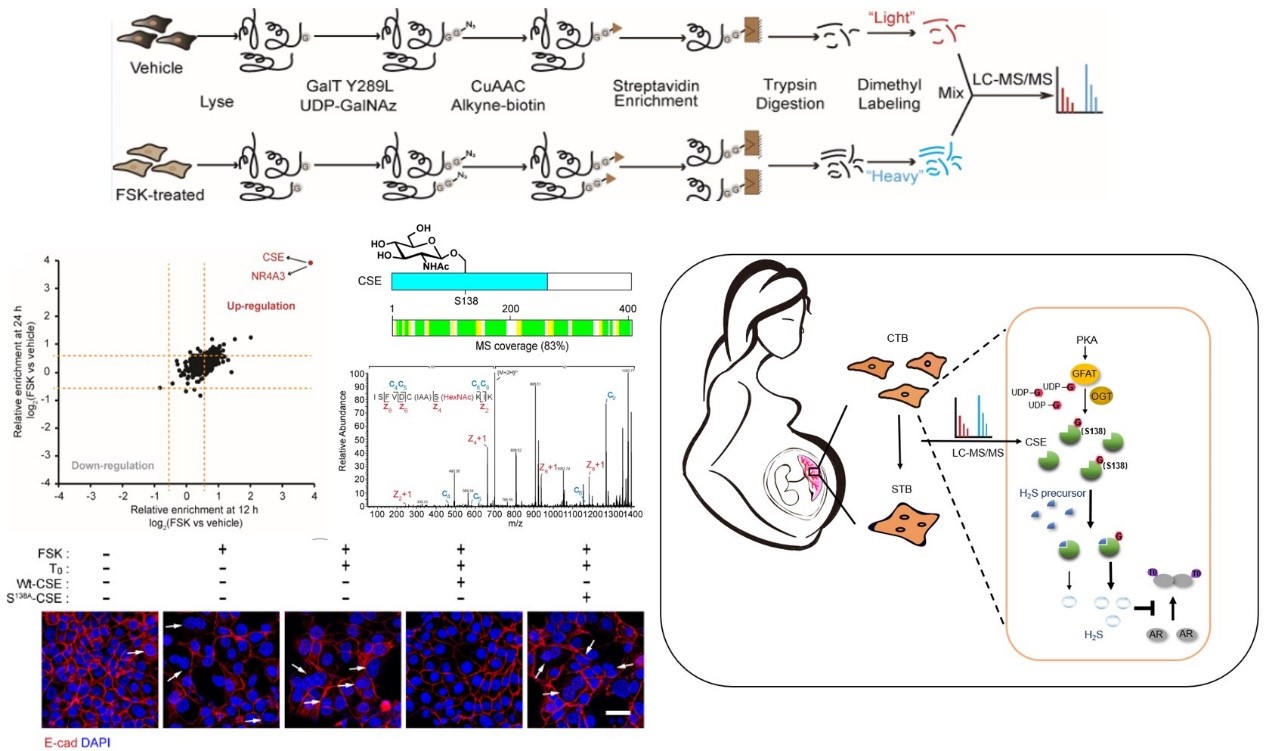
Quantitative chemoproteomics reveals O-GlcNAcylation of Cystathionine γ-lyase (CSE) represses trophoblast syncytialization (Cell Chem Biol 2021)
| Research content and objectives: The primary objective of the research conducted in Dr. Wang's lab is to elucidate the regulatory mechanisms underpinning placental development in humans. This endeavor aims to enhance our comprehension of the pathogenesis of severe pregnancy-associated disorders, such as preeclampsia, recurrent spontaneous abortion, and fetal growth restriction. The overarching goal is to identify pivotal checkpoints in placental development that determine pregnancy outcomes and to pinpoint reliable, specific molecular targets for the early diagnosis and intervention of these related diseases. The specific objectives of this research encompass: - The process of lineage programming in placental trophoblast cells.
- Mechanisms underlying the establishment of uteroplacental-fetal circulation.
- The cellular and molecular mechanisms underlying immune adaptation during pregnancy.
- The pathogenesis of severe pregnancy complications, including preeclampsia and recurrent spontaneous abortion.
Selected publications: - Yu X#, Wu H#, Su J#, Liu X, Liang K, Li Q, Yu R, Shao X, Wang H*, Wang YL*, Shyh-Chang N*. Acetyl-CoA metabolism maintains histone acetylation for syncytialization of human placental trophoblast stem cells. Cell Stem Cell. 2024 Sep 5;31(9):1280-1297.e7.
- Jia W#, Ma L#, Yu X#, Wang F, Yang Q, Wang X, Fan M, Gu Y, Meng R, Wang J, Li YX, Li R*, Shao X*, Wang YL*. Human CD56+CD39+ dNK cells support fetal survival through controlling trophoblastic cell fate: immune mechanisms of recurrent early pregnancy loss. Natl Sci Rev. 2024 Apr 11;11(6):nwae142.
- Zheng W#, Zhang Y#, Xu P, Wang Z, Shao X, Chen C, Cai H, Wang Y, Sun MA, Deng W, Liu F, Lu J, Zhang X, Chen D, Mysorekar IU, Wang H, Wang YL*, Hu X*, Cao B*. TFEB safeguards trophoblast syncytialization in humans and mice. Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2404062121.
- Ma Y#, Yu X#, Ye S#, Li W, Yang Q, Li YX, Wang Y*, Wang YL*. Immune-regulatory properties of endovascular extravillous trophoblast cells in human placenta. Placenta. 2024 Jan;145:107-116.
- Li Y, Li Z, Wang C, Yang M, He Z, Wang F, Zhang Y, Li R, Gong Y, Wang B, Fan B, Wang C, Chen L, Li H, Shi P, Wang N, Wei Z, Wang YL, Jin L, Du P, Dong J, Jiao J. Spatiotemporal transcriptome atlas reveals the regional specification of the developing human brain. Cell. 2023 Dec;186(26):5892-5909.e22.
- Shao X#, Yang Y#, Liu Y#, Wang Y#, Zhao Y, Yu X, Liu J, Li YX, Wang YL*. Orchestrated feedback regulation between melatonin and sex hormones involving GPER1-PKA-CREB signaling in the placenta. J Pineal Res. 2023:e12913.
- Shao X#, Yu W#, Yang Y#, Wang F, Yu X, Wu H, Ma Y, Cao B*, Wang YL*. The mystery of the life tree: the placenta. Biol Reprod. 2022; 25;107(1):301-316.
- Li Y#, Li Z#, Yang M#, Wang F#, Zhang Y#, Li R#, Li Q, Gong Y, Wang B, Fan B, Wang C, Chen L, Li H, Ong J, Teng Z, Jin L*, Wang YL*, Du P*, Jiao J*. Decoding the temporal and regional specification of microglia in the developing human brain. Cell Stem Cell. 2022;29(4):620-634.e6.
- Chen J#, Du L#, Wang F#, Shao X#, Wang X, Yu W, Bi S, Chen D, Pan X, Zeng S, Huang L, Liang Y, Li Y, Chen R, Xue F, Li X, Wang S, Zhuang M, Liu M, Lin L, Yan H, He F, Yu L, Jiang Q, Xiong Z, Zhang L, Cao B*, Wang YL*, Chen D*. Cellular and molecular atlas of the placenta from a COVID-19 pregnant woman infected at mid-gestation highlights the defective impacts on fetal health. Cell Prolif. 2022;55(4):e13204.
- Li G#, Wang Y#, Cao G#, Ma Y, Li YX, Zhao Y*, Shao X*, Wang YL*. Hypoxic stress disrupts HGF/Met signaling in human trophoblasts: implications for the pathogenesis of preeclampsia. J Biomed Sci. 2022;29(1):8.
- Ma Y#, Yu X#, Zhang L#, Liu J, Shao X, Li YX, Wang YL*. Uterine decidual niche modulates the progressive dedifferentiation of spiral artery vascular smooth muscle cells during human pregnancy. Biol Reprod. 2021 Mar 11;104(3):624-637.(Editorial Choice; Faculty Opinions)
- Shao X#, Cao G#, Chen D#, Liu J, Yu B, Liu M, Li YX, Cao B*, Sadovsky Y*, Wang YL*. Placental trophoblast syncytialization potentiates macropinocytosis via mTOR signaling to adapt to reduced amino acid supply. Proc Natl Acad Sci U S A. 2021;118(3):e2017092118.
- Wang F#, Jia W#, Fan M#, Shao X#, Li Z, Liu Y, Ma Y, Li YX, Li R*, Tu Q*, Wang YL*. Single-cell immune landscape of human recurrent miscarriage. Genomics Proteomics Bioinformatics. 2021;S1672-0229(21)00003-6. (Editorial Highlight)
- Liu J#, Shao X#, Qin W#, Yanling Zhang#, Dang F, Yang Q, Yu X, Li YX, Chen X, Wang C*, Wang YL*. Quantitative chemoproteomics reveals O-GlcNAcylation of Cystathionine γ-lyase (CSE) represses trophoblast syncytialization. Cell Chem Biol. 2021;S2451-9456(21)00050-7. (Cover story)
- Ma Y#, Yang Q#, Fan M#, Zhang L, Gu Y, Jia W, Li Z, Wang F, Li YX, Wang J, Li R*, Shao X*, Wang YL*. Placental endovascular extravillous trophoblasts (enEVTs) educate maternal T-cell differentiation along the maternal-placental circulation. Cell Prolif. 2020;53(5):e12802.
- Shao X#, Wang Y#, Liu Y, Guo X, Li D, Huo R, Jia W, Cao G, Li YX, Liu M, Sha J, Zhao Y, Wang YL*. Association of imbalanced sex hormone production with excessive procoagulation factor SerpinF2 in preeclampsia. J Hypertens. 2019;37:197–205
- Shao X#, Liu Y#, Liu M, Wang Y, Yan L, Wang H, Ma L, Lia YX, Zhao Y*, Wang YL*. Testosterone represses estrogen signaling via upregulating mir-22: mechanism for imbalanced steroid hormone production in preeclampsia. Hypertension. 2017;69:721-730. (Editorial highlight)
- Xu P#, Zhao Y#, Liu M, Wang Y, Wang H, Li YX, Zhu X, Yao Y, Wang H, Qiao J, Ji L*, Wang YL*. Variations of micrornas in human placentas and plasma from preeclamptic pregnancy. Hypertension. 2014;63:1276-1284.
- Ji L#, Brkić J#, Liu M#, Fu G, Peng C*, Wang YL*. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Asp Med. 2013;34:981-1023.
|

