| Dr. Wei, the principal investigator of the lab, has constructed and optimized a series of efficient nano-delivery systems for small molecule drugs, RNAs, and CRISPR/Cas systems. To date, she has published over 40 papers in high-impact journals, including Nat Nanotechnol., Nat Commun. and Adv Mater. with more than 5800 total citations and an H-index of 31 (Google Scholar). She has 13 Chinese and international patent applications (9 of which have been granted), and several patents have been licensed to a bio-company for clinical translation. Dr. Wei currently serves as the Young Editorial Board Member of the journal of “Exploration”, the Editorial Board Member of the “Journal of Functional Biomaterials”, the Young Editorial Board Member of the journal of “iLiver”, and is a member of the Nano-oncology Committee of the Chinese Anti-Cancer Association. In addition, she serves as an invited reviewer for Nano Today, Angewandte Chemie, Advanced Science, and other prestigious journals. Our research group focuses on the key scientific questions in the treatment of deadly human diseases (such as cancer and genetic diseases) and the challenges of in vivo delivery of small molecule drugs and biomacromolecules (mRNA and proteins), using multidisciplinary technologies including chemistry, material science, biology, and medicine to design and develop novel nano-delivery technology platforms to improve drug loading capacity, increase in vivo stability, and enhance endosomal escape capacity through screening and optimization of delivery vectors. We aim to provide new methods and insights for the treatment of diseases that threaten human life and health. 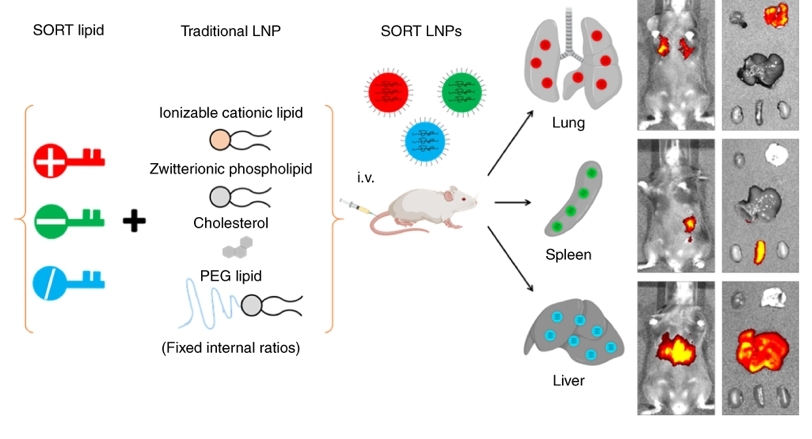
Development of SORT technology for organ-specific delivery of RNA in vivo.
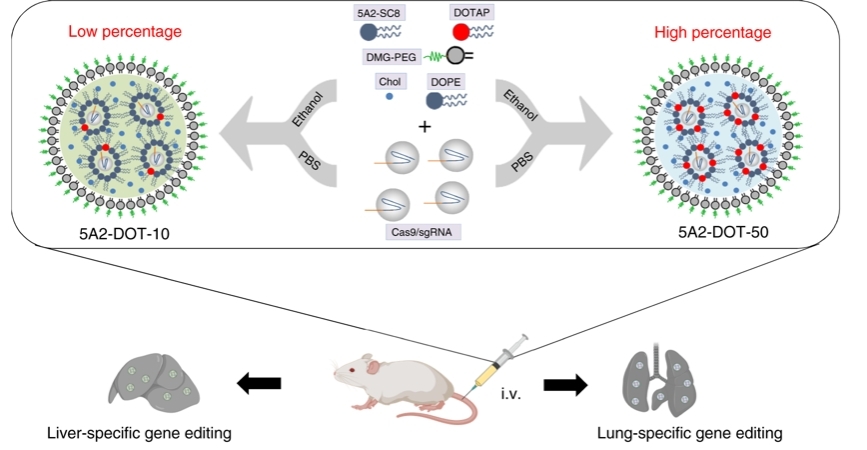
Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing
| Plain english:
Our research group focuses on the key scientific questions in the treatment of deadly human diseases (such as cancer, genetic diseases, etc.) and utilizes multidisciplinary technologies including chemistry, material science, biology, and medicine to design and develop novel nano-delivery technology platforms for highly efficient and targeted delivery of small molecule drugs and biomacromolecules (such as RNA, proteins, and gene editing systems), and to broaden and deepen their applications in the biomedical field. The research fields include: (1) Development of organ/cell type targeted delivery systems; (2) Development of novel delivery systems for gene editing and gene editing-based therapeutics; (3) Targeted gene therapy for fatal human diseases (e.g., tumors, metabolic diseases, and genetic diseases). Selected publications: - Lin Y, Cheng Q*, Wei T*. Surface Engineering of Lipid Nanoparticles: Targeted Nucleic Acid Delivery and Beyond. Biophys Rep. 2023, 9(5): 255-278. (Invited Review)
- Wei T#, Sun Y#, Cheng Q#, Chatterjee S, Traylor Z, Johnson LT, Coquelin ML, Wang J, Torres MJ, Lian X, Wang X, Xiao Y, Hodges CA, Siegwart DJ. Lung SORT LNPs enable precise homology-directed repair mediated CRISPR/Cas genome correction in cystic fibrosis models. Nat Commun. 2023, 14(1):7322.
- Zong Y, Lin Y, Wei T*, Cheng Q*. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv Mater. 2023, https://doi.org/10.1002/adma.202303261.
- Wei T*, Tao W*, Cheng Q*. Lipid nanoparticles for mRNA therapy: recent advances in targeted delivery. Life Medicine. 2022, https://doi.org/10.1093/lifemedi/lnac004.
- Wei T#, Cheng Q#, Min YL, Olson EN, Siegwart DJ*. Systemic nanoparticle delivery of CRISPR/Cas9 ribonucleoproteins for effective tissue-specific genome editing. Nat Commun. 2020, 11(1):3232. (Highly cited paper)
- Cheng Q#, Wei T#, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ*. Selective ORgan Targeting (SORT) nanoparticles for tissue specific mRNA delivery and CRISPR/Cas gene editing. Nat Nanotechnol. 2020, 15(4):313. (Highly cited paper)
- Wei T, Cheng Q, Farbiak L, Anderson DG*, Langer R*, Siegwart DJ*. Delivery of Tissue-Targeted Scalpels: Opportunities and Challenges for in Vivo CRISPR/Cas-Based Genome Editing. ACS Nano. 2020, 14(8):9243.(Invited Review)
- Liu J#, Wei T#, Zhao J, Huang Y, Deng H, Kumar A, Wang C, Liang Z, Ma X*, Liang X-J*. Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance. Biomaterials. 2016, 91:44.
- Wei T, Chen C, Liu J, Liu C, Posocco P, Liu X, Cheng Q, Huo S, Liang Z, Fermeglia M, Pricl S, Liang X-J*, Rocchi P, Peng L*. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci U S A. 2015, 112(10):2978.
- Wei T#, Liu J#, Ma H, Cheng Q, Huang Y, Zhao J, Huo S, Xue X, Liang Z, Liang X-J*. Functionalized Nanoscale Micelles Improve Drug Delivery for Cancer Therapy in Vitro and in Vivo. Nano Lett. 2013, 13(6):2528.
(# Co-first, * Corresponding) |

