| The research areas of our laboratory cover basic research and application of reproductive, developmental biology and stem cells. We have derived and established embryonic stem (ES) cell lines from mouse, rat, monkey, and human, and have set up stable technical platforms for cell reprogramming, including somatic cell nuclear transfer and induced pluripotent stem (iPS) cell technology, for several species. We generated viable mice from iPS cells through tetraploid complementation, demonstrating that iPS cells have the similar pluripotency to that of ES cells. We discovered and demonstrated a key gene-regulation region that may serve as a molecular marker for evaluating the pluripotency level of stem cells, and investigated its regulatory mechanism. We demonstrated that mice generated from iPS cells have the same physiological functions as that from ES cells but are prone to tumorigenesis. In addition, we derived mammalian haploid stem cells and explored their applications in reproduction, development, and genetic modification. Using genetically modified haploid ES cells we generated transgenic mice and rats, and carried out genetic screening in rats, providing important new tools for studying mammalian reproduction and genetics. On the basis of haploid stem cells, we generated world-first mammalian diploid hybrid ES cells of phylogenetically distal species, which will be a valuable tool in studying X-chromosome inactivation (XCI) and genes with functional differences between species. By combining genome editing and haploid embryonic stem cell technologies, we produced healthy and fertile bimaternal mice, and, for the first time, full-term bipaternal mice, which will be valuable to uncover the functions of genomic imprinting, and to improve assisted reproduction in diverse mammalian species. We identified a long noncoding RNA, LincGET, as one of the earliest known lineage regulators to bias cell fate in mammalian 2-cell embryos. We also discovered that overcoming the genomic imprinting barrier can improve the efficiency of mammalian cloning. In addition, we established genetic modification systems for several species including mouse, rat, pig, and non-human primate. We derived rat ES cell lines with germline-competent, and simultaneously generated multiple gene mutations in rat using CRISPR-Cas system. We also efficiently generated biallelic vWF-knockout pigs and p53 gene biallelic mutant Cynomolgus monkey via one-step methods, demonstrating that CRISPR technology is applicable to genome engineering of large animals, and providing important tools for preparing animal models for human diseases. In clinical application of stem cells, based on strict quality system, the National Stem Cell Resource Center (formerly Beijing Stem Cell Bank) has established and stored a variety of clinical-grade embryonic stem cell lines and various functional cell lines, which have been quality assessed by the National Institutes for Food and Drug Control, and have been used in a number of clinical studies of stem cells. Starting from July 2020, the National Stem Cell Resource Center has become an independent research department of the Institute of Zoology, Chinese Academy of Sciences (CAS). 
Mouse generated from iPS cells via tetraploid complementation demonstrated that iPS cells can attain true pluripotency similar to that of ES cells.
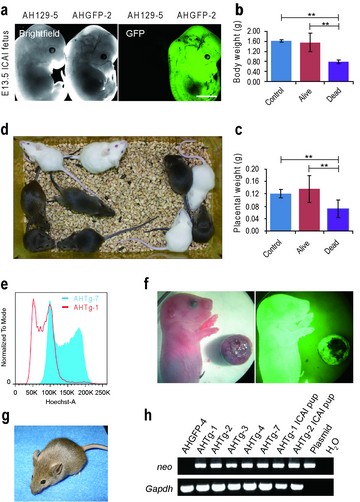
Generation of live transgenic mice from androgenetic haploid ES cells not only demonstrated the developmental pluripotency of androgenentic haploids ES cells, but also provides a new tool for quickly producing animal models of recessive traits and for assisted reproduction.
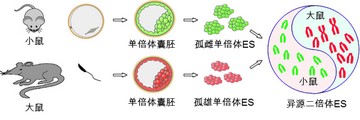
Generation of mouse-rat allodiploid embryonic stem cells, which will be a valuable tool in studying XCI and genes with functional differences between species.
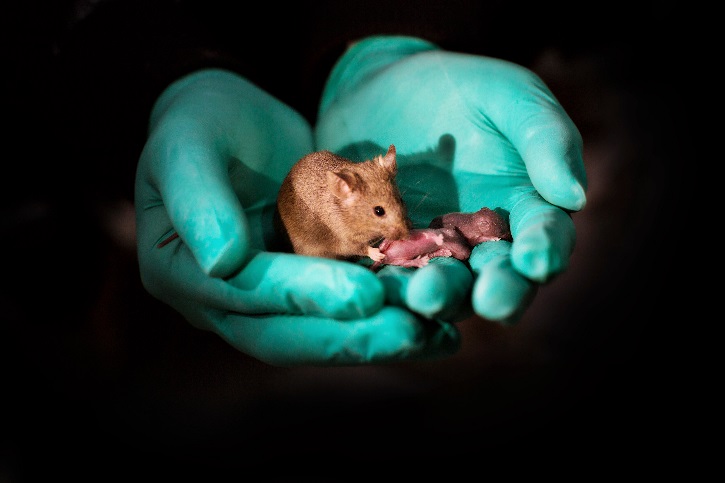
A bimaternal mouse (born to two mothers) tends to her own pups.
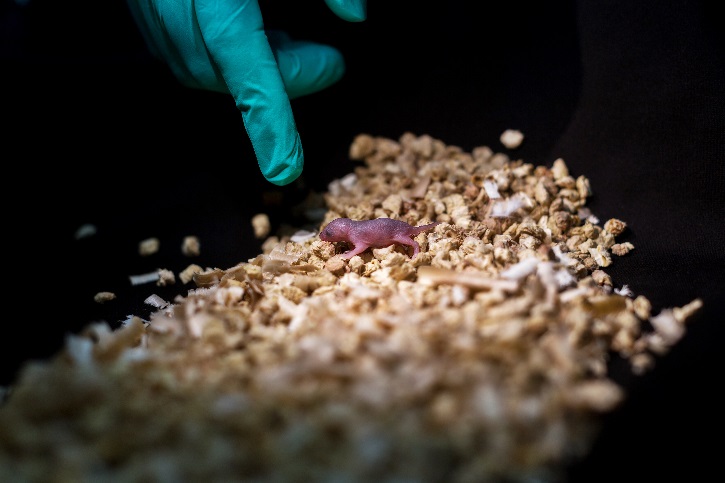
A bipaternal mouse pup (born to two fathers)
| Plain english:
Basic research and application of reproductive, developmental biology and stem cells. Selected publications: - Wang LB#, Li ZK#, Wang LY#, Xu K#, Ji TT#, Mao YH, Ma SN, Liu T, Tu CF, Zhao Q, Fan XN, Liu C, Wang LY, Shu YJ, Yang N, Zhou Q*, Li W*. A sustainable mouse karyotype created by programmed chromosome fusion. (2022) Science 377(6609):967-975. doi: 10.1126/science.abm1964.
- Wang LY#, Li ZK#, Wang LB#, Liu C#, Sun XH, Feng GH, Wang JQ, Li YF, Qiao LY, Nie H, Jiang LY, Sun H, Xie YL, Ma SN, Wan HF, Lu FL*, Li W*, Zhou Q*. Overcoming Intrinsic H3K27me3 Imprinting Barriers Improves Post-implantation Development after Somatic Cell Nuclear Transfer. (2020) Cell Stem Cell 27(2):315-325.e5. doi: 10.1016/j.stem.2020.05.014.
- Wang J#, Wang L#, Feng G#, Wang Y#, Li Y, Li X, Liu C, Jiao G, Huang C, Shi J, Zhou T, Chen Q, Liu Z, Li W*, Zhou Q*. Asymmetric Expression of LincGET Biases Cell Fate in Two-Cell Mouse Embryos. (2018) Cell 175(7):1887-1901.e18. doi: 10.1016/j.cell.2018.11.039.
- Li ZK#, Wang LY#, Wang LB#, Feng GH#, Yuan XW#, Liu C, Xu K, Li YH, Wan HF, Zhang Y, Li YF, Li X, Li W*, Zhou Q*, Hu BY*. Generation of Bimaternal and Bipaternal Mice from Hypomethylated Haploid ESCs with Imprinting Region Deletions. (2018) Cell Stem Cell 23(5):665-676.e4. doi: 10.1016/j.stem.2018.09.004.
- Li X#, Cui XL#, Wang JQ #, Wang YK, Li YF, Wang LY, Wan HF, Li TD, Feng GH, Shuai L, Li ZK, Gu Q, Hao J, Wang L, Zhao XY, Liu ZH, Wang XJ, Li W*, Zhou Q*. Generation and Application of Mouse-Rat Allodiploid Embryonic Stem Cells. (2016) Cell 164(1–2): 279–292. doi: 10.1016/j.cell.2015.11.035.
- Zhou Q#, Wang M#, Yuan Y#, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y, Feng G, Shi Q, Zhao XY*, Sha J*, Zhou Q*. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. (2016) Cell Stem Cell 18(3):330-340. doi: 10.1016/j.stem.2016.01.017.
- Wan HF#, Feng CJ#, Teng F#, Yang SH#, Hu BY, Niu YY, Xiang P, Fang WZ, Ji WZ, LI W*, Zhao XY*, Zhou Q*. One-step generation of p53 gene biallelic mutant Cynomolgus monkey via the CRISPR/Cas system. (2015) Cell Research 25(2):258-261. doi: 10.1038/cr.2014.158.
- Li W#, Li X#, Li TD#, Jiang MG#, Wan HF, Luo GZ, Feng CJ, Cui XL, Teng F, Yuan Y, Zhou Q, Gu Q, Shuai L, Sha JH, Xiao YM, Wang L, Liu ZH, Wang XJ, Zhao XY, Zhou Q*. Genetic modification and screening in the rat using haploid embryonic stem cells. (2014) Cell Stem Cell 14(3):404-414. doi: 10.1016/j.stem.2013.11.016.
- Li W, Teng F, Li TD, Zhou Q*. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. (2013) Nat Biotechnol. 31(8):684-686. doi: 10.1038/nbt.2652.
- Li W#, Shuai L#, Wan HF#, Dong MZ#, Wang M, Sang LS, Feng CJ, Luo GZ, Li TD, Li X, Wang LB, Zheng QY, Sheng C, Wu HJ, Liu ZH, Liu L, Wang L, Wang XJ, Zhao XY*, Zhou Q*. Androgenetic haploid embryonic stem cells produce live transgenic mice. (2012) Nature 490(7420):407-411. doi: 10.1038/nature11435.
- Liu L#, Luo GZ#, Yang W#, Zhao X#, Zheng Q, Lv Z, Li W, Wu HJ, Wang L, Wang XJ*, Zhou Q*. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. (2010) J Biol. Chem. 285(25):19483-19490. doi: 10.1074/jbc.M110.131995.
- Zhao XY#, Li W#, Lv Z#, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, Zeng FY*, Zhou Q*. iPS cells produce viable mice through tetraploid complementation. (2009) Nature 461(7260):86-90. doi: 10.1038/nature08267.

An imprinted genomic region encoding a cluster of microRNAs is positively correlated with the pluripotency levels of iPS cells, and thus can serve as the molecular marker for evaluating the pluripotency level of stem cells at an early stage.
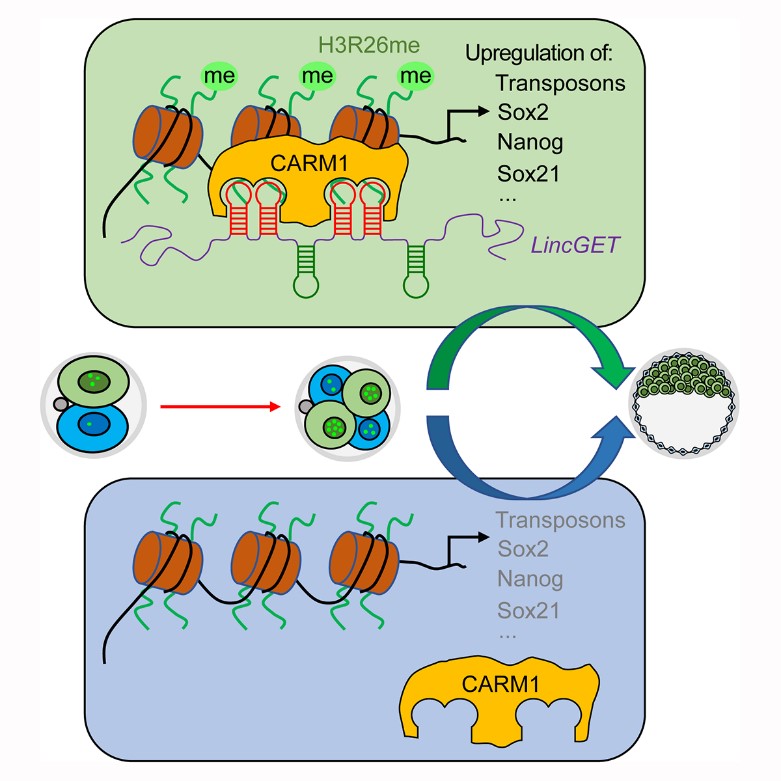
LincGET is asymmetrically expressed in the nucleus of two to four-cell mouse embryos, and regulates cell fate selection through CARM1.

One-step generation of multiple gene mutations in rat and p53 gene biallelic mutant monkey via CRISPR/Cas system.
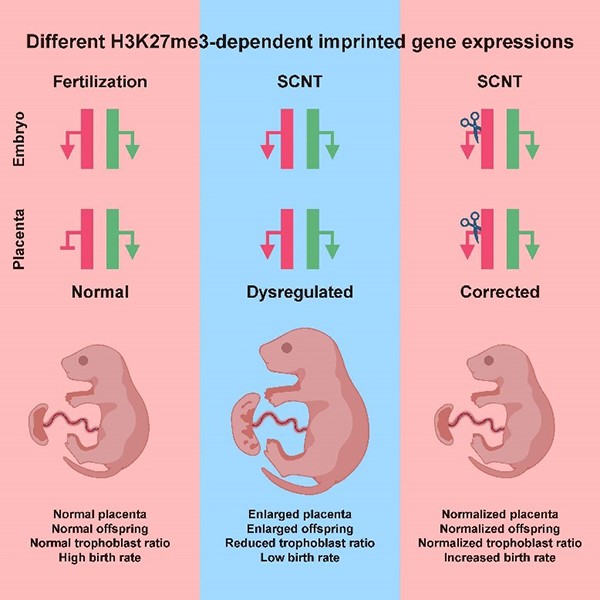
Overcoming intrinsic H3K27me3 imprinting barriers improves efficiency of somatic cell nuclear transfer.
|

